AWARD YEAR
2021
CATEGORY
Body
GOALS
Good Health & Well-being
KEYWORDS
low-cost, ventilator, covid-19
COUNTRY
United States of America
DESIGNED BY
University of Minnesota researchers
WEBSITE
https://twin-cities.umn.edu/news-events/fda-authorizes-first-its-kind-low-cost-ventilator-developed-university-minnesota
Coventor
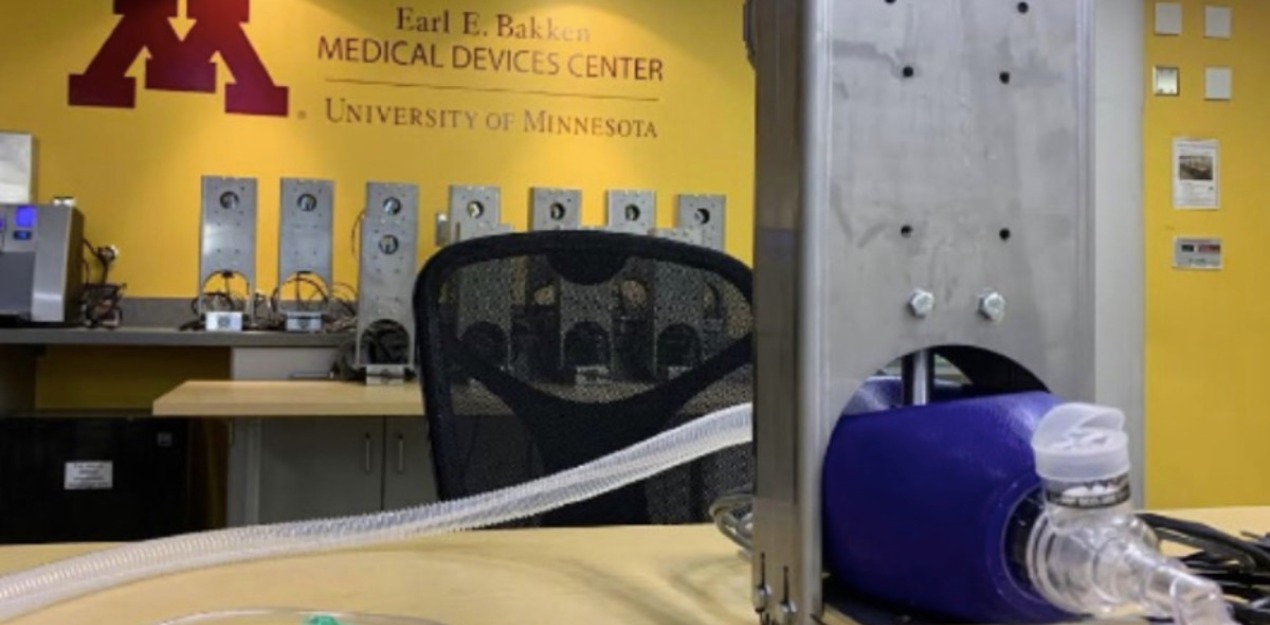
FDA authorizes first-of-its-kind, low-cost ventilator developed by University of Minnesota
The U.S. Food and Drug Administration (FDA) today authorized the production and use of a low-cost ventilator first conceived by a team of University of Minnesota researchers and an alumnus. Known as the Coventor, this ventilator is the first-of-its-kind authorized for use under the FDA's Emergency Use Authorization for the COVID-19 outbreak. Ventilators are used in a clinical setting to assist patients with increasing their blood oxygen levels. They have become a critical treatment device for COVID-19 and were used previously to support patients with pneumonia and acute respiratory distress. Ventilators are in short supply nationwide as hospitals respond to the COVID-19 pandemic. Richardson and a U of M research team developed and designed the Coventor, a low-cost backup alternative for physicians to use. Financial and in-kind support for this initiative has been provided by Midwest companies Digi-Key, MGC Diagnostics and Protolabs, and Teknic, Inc. from New York.